Phosphoinositide Signaling in Cell Biology and Cancer
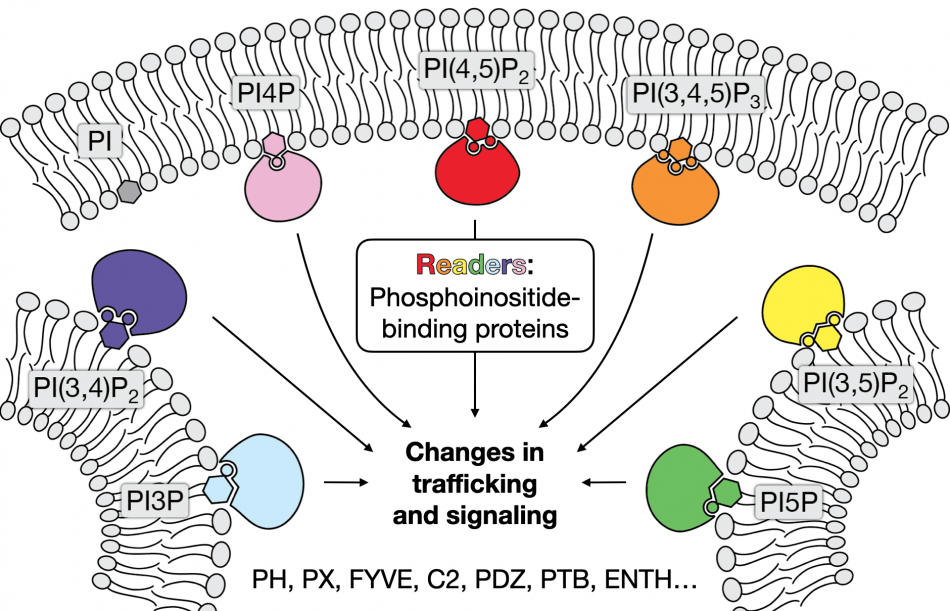
Phosphoinositides are a key group of signaling lipids. This lipid family comprises seven members defined by their head group phosphorylation pattern, set by a group of “writer” and “eraser” enzymes (lipid kinases and phosphatases). How these enzymes are regulated in space and time to establish the precise distribution of phosphoinositides within distinct organelle membranes, termed the phosphoinositide code of membrane identity, is a major open question in the field. One avenue of research we are pursuing is to reveal such mechanisms for a single such enzyme, called a phosphatidylinositol 4-kinase, in the plasma membrane.
A critical function of phosphoinositides is to act as molecular signposts on specific organelle membranes. In this role, these lipids act as ligands for “reader” proteins, which bind to the lipid head groups and subsequently initiate or propagate signaling pathways from those precise membrane locations. To understand how the phosphoinositide code contributes to important signaling events in fundamental cell biology and in cancer, we have focused our efforts on a novel reader protein called PLEKHA4 and its paralog PLEKHA5. The expression of these proteins is highly upregulated in many cancers, with the levels of PLEKHA4 being the most elevated in melanoma, the deadliest form of skin cancer. Prior to our work, its molecular and cellular functions remained completely unknown.
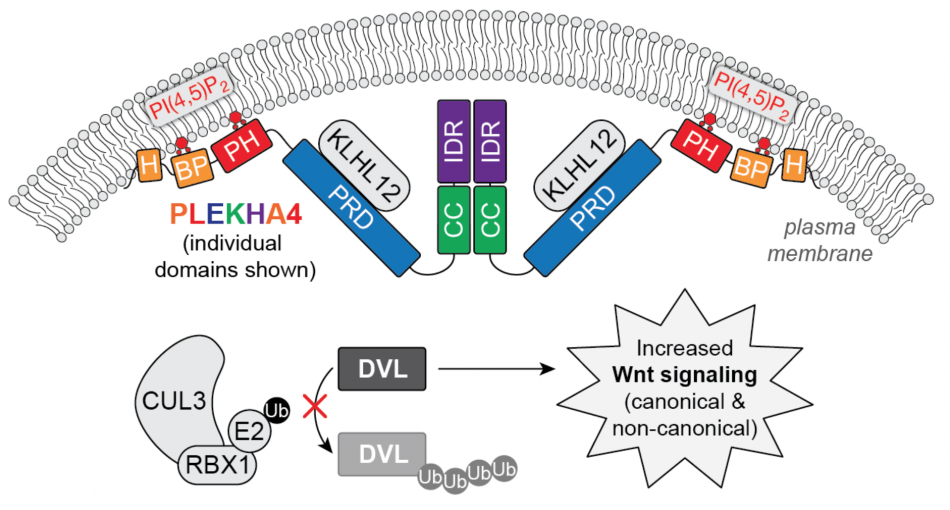
We have begun to examine the mechanisms by which PLEKHA4 functions in normal physiological settings and in cancer cells. Our studies combine biochemistry, molecular and cell biology, live-cell confocal microscopy, and animal models in the fruit fly Drosophila melanogaster and in mice. In short, we have revealed a deep connection between the interactions of PLEKHA4 with its lipid and protein binding partners and the regulation of ubiquitination machinery and Wnt signaling pathways that control cell fate in development and proliferation in cancer. Our recent discovery that PLEKHA4 is required for tumor proliferation in mouse models of melanoma establishes this phosphoinositide reader protein as a promising new potential drug target in melanoma. We are currently pursuing the translation of these discoveries as well as further elucidation of mechanisms by which PLEKHA4, PLEKHA5, and other related proteins control diverse physiological and pathological signaling events.
Key Publications
Luan L, Cao X, Baskin JM. “Inhibition of SQSTM1/p62 oligomerization and Keap1 sequestration by the Cullin-3 adaptor SHKBP1.” bioRxiv(2025). doi: https://doi.org/10.1101/2025.01.21.634088.
Cao X, Huang S, Wagner M, Cho Y-T, Chiu D-C, Wartchow KM, Lazarian A, McIntire LB, Smolka MB, Baskin JM. “A phosphorylation-controlled switch confers cell cycle-dependent protein relocalization.” Nat Cell Biol (2024) 26, 1804–1816.
Sun H, Shami Shah A, Chiu DC, Bonfini A, Buchon N, Baskin JM. “Wnt/β-catenin signaling within multiple cell types dependent upon kramer regulates Drosophila intestinal stem cell proliferation.” iScience (2024) 27, 6, 110113.
Cao X, Shami Shah A, Sanford EJ, Smolka MB, Baskin JM. “Spatial regulation of the anaphase-promoting complex/cyclosome by a microtubule adaptor.” ACS Chem Biol (2022) 17, 9, 2605–2618.
Batrouni AG*, Bag N*†, Phan H, Baird BA, and Baskin JM†. “A palmitoylation code controls PI4KIIIα complex formation and PI(4,5)P2 homeostasis at the plasma membrane.” J Cell Sci (2022) 135, 5, jcs259365. *Equal contribution; †Co-corresponding authors. Part of special issue on Cell Biology of Lipids.
Shami Shah A, Cao X, White AC, Baskin JM. “PLEKHA4 Promotes Wnt/β-catenin Signaling-Mediated G1/S Transition and Proliferation in Melanoma.” Cancer Res (2021) 81, 2029–43.
Shami Shah A, Batrouni AG, Kim DS, Punyala A, Cao W, Han C, Goldberg ML, Smolka MB, and Baskin JM. “PLEKHA4/kramer Attenuates Dishevelled Ubiquitination to Modulate Wnt and Planar Cell Polarity Signaling.” Cell Rep (2019), 27, 7, 2157–70.

